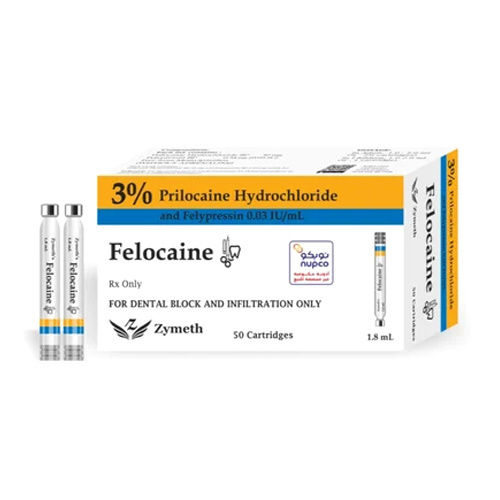
Prilocaine Hydrochloride Injection
Product Details:
- Drug Type Injection
- Physical Form Liquid
- Dosage As per the doctor's advice
- Dosage Guidelines As per the doctor's advice
- Storage Instructions Keep at cool and Dry Place
- Click to View more
X
Prilocaine Hydrochloride Injection Price And Quantity
- 100 Box
Prilocaine Hydrochloride Injection Product Specifications
- Liquid
- Keep at cool and Dry Place
- As per the doctor's advice
- Injection
- As per the doctor's advice
Prilocaine Hydrochloride Injection Trade Information
- 5000 Box Per Month
- 10 Days
Product Description
| Grade | Pharma Grade |
| Grade Standard | USP |
| Usage/Application | Pharmaceutical |
COMPOSITION
Each mL contains :
- Prilocaine Hydrochloride BP : 30 mg
- Felypressin BP : 0.54 g (0.03 IU)
- Free from Methylparaben (WITHOUT ADRENALINE)
Each 1.8 mL Contains:
- Prilocaine Hydrochloride BP 54 mg
- Felypressin BP 0.972 g (0.054 IU)
THERAPEUTIC ACTION
- Local Anesthesia for Dental Procedures by Nerve block or infiltration techniques.
INDICATION
- Felocaine is indicated for the production of local anaesthesia in routine dental procedures and oral surgery by means of infiltration and nerve block techniques
CONTRAINDICATION
- Allergy or hypersensitivity to amide type local anaesthetics or other components of the injection solution which may be present. Detection of suspected sensitivity by skin testing is of limited value.
- Congenital or idiopathic methaemoglobinaemia.
- Local anaesthetic techniques must not be used when there is inflammation and/or sepsis in the region of the proposed injection.
DOSAGE
- In Adults: 1.0 - 5.0 mL (0.5 to 2.5 Cartridges)
- In Children: 1.0 - 2.0 mL (0.5 to 1 Cartridge)
PRESENTATIONS
- 50 dental Cartridges x 1 Box. (available in cardboard boxes containing 5 blisters of 10 x 1.8 mL cartridges)
Enter Buying Requirement Details
Other Products in 'Pharmaceutical Injection' category
Back to top